|
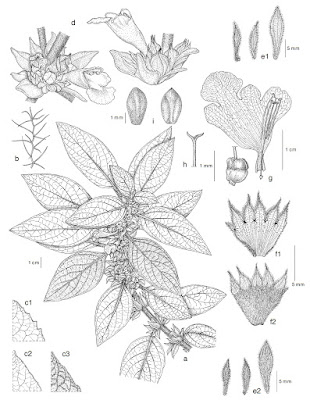
Representatives of diademichthyine clingfishes.
A Rhinolepadichthys lineatus (Oman); B Rhinolepadichthys geminus (Anilao, Philippines);
C Rhinolepadichthys geminus (Okinoerabu Islands, Amami Islands, Japan); D Discotrema crinophilum (Amami-oshima Island, Amami Islands, Japan: KPM-NR 78755);
E Lepadichthys frenatus (Lord Howe Island, Australia); F Diademichthys lineatus (Lembeh Strait, Indonesia: KPM-NR 147468).
in Fujiwara, Motomura, Summers & Conway, 2024. All images except F with sides reversed. photos by J. Randall, J. Eyre and K. Uehara. |
Abstract
Rhinolepadichthys, a new genus of the gobiesocid subfamily Diademichthyinae, is described for the “Lepadichthys” lineatus complex (including Rhinolepadichthys geminus comb. nov., R. heemstraorum comb. nov., R. lineatus comb. nov., and R. polyastrous comb. nov.). Detailed investigation of external morphology and osteological anatomy of the new genus and related genera suggests that Rhinolepadichthys represents the sister genus to Discotrema, based on the following putative synapomorphies: (1) presence of a hardened (potentially keratinized) cap on the surface of at least some disc papillae (vs. surface of disc papillae soft, without hardened cap); and (2) the anterolateral part of the ventral postcleithrum extended anteriorly as a well-developed rod-like process, its tip close to the base of pelvic-fin soft ray 4 (vs. only weakly pointed, or irregular). Compared with Discotrema, Rhinolepadichthys gen. nov. is distinguished by the presence of a row of 8–12 large papillae on the inner surface of the upper and lower lips (vs. inner surface of lips smooth, without distinct papillae); the absence (vs. presence) of a well-developed lateral process on the pterotic immediately posterior to the opening of the otic canal; the presence (vs. absence) of gill rakers on the anterior edge of ceratobranchials 1–3; the presence (vs. absence) of gill rakers on the posterior edge of ceratobranchial 4; having the upper pharyngeal teeth arranged in a loose patch on the ventral surface of the pharyngobranchial 3 toothplate, with tooth tips directed posteroventrally (vs. arranged in a single row along posteroventral edge of the pharyngobranchial 3 toothplate, with tooth tips directed posteriorly); features of the adhesive disc, including outline of disc papillae roughly hexagonal or ovoid and with a flattened surface (vs. outline circular, at least some with raised, dome-like surface); the absence (vs. presence) of a deep cavity at the center of disc region C; the absence (vs. presence) of three paired and one median cluster of small papillae (reminiscent of bunches of grapes) across the surface of the adhesive disc; and having the ventral postcleithrum entire, not divided into two separate, articulating elements (vs. ventral postcleithrum divided into an anterior and posterior element, separated via a specialized joint). Reexamination of materials of the poorly known genus Unguitrema, considered a close relative of Discotrema, revealed no morphological differences between the two genera. Unguitrema therefore represents a junior synonym of Discotrema.
Keywords: Clingfishes, Indo-Pacific, morphology, taxonomy, Teleostei
 |
Representatives of diademichthyine clingfishes.
A Rhinolepadichthys lineatus (Oman: J. Randall); B Rhinolepadichthys geminus (Anilao, Philippines: J. Eyre);
C Rhinolepadichthys geminus (Okinoerabu Islands, Amami Islands, Japan: K. Uehara); D Discotrema crinophilum (Amami-oshima Island, Amami Islands, Japan: KPM-NR 78755, K. Uchino);
E Lepadichthys frenatus (Lord Howe Island, Australia: J. Eyre); F Diademichthys lineatus (Lembeh Strait, Indonesia: KPM-NR 147468, K. Uchino).
All images except F with sides reversed. |
Rhinolepadichthys gen. nov.
Included species: The genus contains the following four valid species, previously included in the “Lepadichthys” lineatus complex by Fujiwara and Motomura (2021): Rhinolepadichthys geminus (Fujiwara and Motomura, 2021) comb. nov., Rhinolepadichthys heemstraorum (Fujiwara and Motomura, 2021) comb. nov., Rhinolepadichthys lineatus (Briggs, 1966) comb. nov., and Rhinolepadichthys polyastrous (Fujiwara and Motomura, 2021) comb. nov.
Etymology: The suffix rhino-, meaning nose, in combination with Lepadichthys, a genus of the Diademichthyinae. In reference to the pointed snout in members of this genus, which distinguishes the new genus from Lepadichthys (sensu stricto). Gender masculine.
Discotrema Briggs, 1976
Included species: The genus contains the following four valid species, Discotrema crinophilum Briggs, 1976, Discotrema monogrammum Craig & Randall, 2008, Discotrema nigrum (Fricke, 2014), comb. nov. (validity tentative, see below), and Discotrema zonatum Craig & Randall, 2008.
Kyoji Fujiwara, Hiroyuki Motomura, Adam P. Summers and Kevin W. Conway. 2024. A New Generic Name for the “
Lepadichthys”
lineatus complex with A Rediagnosis of
Discotrema, a senior synonym of
Unguitrema, and Comments on their phylogenetic relationships (Gobiesocidae: Diademichthyinae).
Vertebrate Zoology. 74: 279-301. DOI:
10.3897/vz.74.e113955